
Growing up in a drought-prone area that is also the home to some of the best beaches on the planet, I grew up with harsh water restrictions and vast oceans next door, so the case for desalination was always close to home for me. Like many others, my knowledge of desalination techniques was poor, outside of boiling water and collecting steam I had little understanding of how to extract salt from water efficiently. Since working in the industry I have been exposed to the vast technologies out there which led me to create this quick guide on the various methods.
Distillation, Reverse Osmosis (RO), Freeze-Thaw, Electrodialysis membrane and Microbial desalination are the five recognized types of desalination. Although Distillation and RO dominate global usage, the remaining three methods warrant explanation due to their unique exploitation of chemical properties.
Desalination is the process of getting salt out of the water, whether that is seawater or brackish water, the process is the same. As this is simply a process of getting a solute (salt) out of a solvent (water), the general methodology goes back to basic chemistry principles, however, some methods are much more efficient than others, particularly as technology has improved.
Distillation
Distillation is perhaps the most widely recognized of all desalination methods namely because everyone has at some point in their life, boiled water and noticed the steam rise off the surface and the volume of water slowly decrease. This very principle of evaporation into steam and then condensation into water is what distillation is based around, the water is boiled, the steam captured and then the steam is condensed into pure water.

Although the general principle around distillation is quite simple there are various types of distillation that can affect the efficiency and general nature of the process.
Multi-stage flash distillation
Multi-Stage Flash Distillation more commonly referred to as MSF is the most common and efficient form of distillation. The process works by evaporating pure water from salt water through a series of “Flash Evaporations” which take place after they go through a thermostatic expansion valve, both of those terms are explained in the glossary of this webpage.
The energy released from each subsequent flash evaporation utilizes energy released from the condensation of the water vapour from the previous stage. This leads to an overall increase in the efficiency of the process as less energy is lost to the atmosphere.
Solar Distillation
Solar Distillation involves the water being evaporated from the sun’s energy, there are two sub-types: Firstly, using solar panels to collect electrical energy to then power the desalination process. The second type uses the sun’s energy to heat the water directly, in the exact same way water is evaporated into clouds in nature, this is known as solar thermal powered desalination.
Natural Evaporation
Natural Evaporation follows similar principles to solar thermal powered desalination, however, it is performed in a closed-off area like a greenhouse, where the humidity is much higher than the outside environment. This increased humidity leads to a much higher rate of evaporation and condensation. A clever way to induce natural evaporation when hiking or camping is explained here.

Vacuum distillation
Vacuum distillation occurs under reduced atmospheric pressure, this lower pressure drops the required temperature to evaporate water. As liquids boil/evaporate when their vapour pressure equals the surrounding ambient temperature, a decreased ambient/atmospheric temperature means that the water boils at a lower temperature. This drop in target temperature requires less energy to reach and thus is more efficient than distillation at normal atmospheric pressure.
Multiple-effect distillation
Multiple-effect distillation is a multi-step process that is theoretically very efficient but difficult to execute at scale due to practical limitations. The process works with incoming water being sprayed onto heated pipes which convert the water to steam on impact steam. As the hot steam rises it is captured and used to help heat the next set of pipes which also condenses the water, these pipes are then used to evaporate the next batch of seawater and the process continues.
Vapor-compression distillation
Vapor-compression evaporation functions by compressing the vapour present above seawater. This compressed vapour is now hotter than its surroundings and is used to heat the rest of the seawater to be desalinated. As the amount of vapour captured and compressed controls the quantity of seawater to be processed it is generally a small-scale operation.
Reverse-Osmosis Desalination
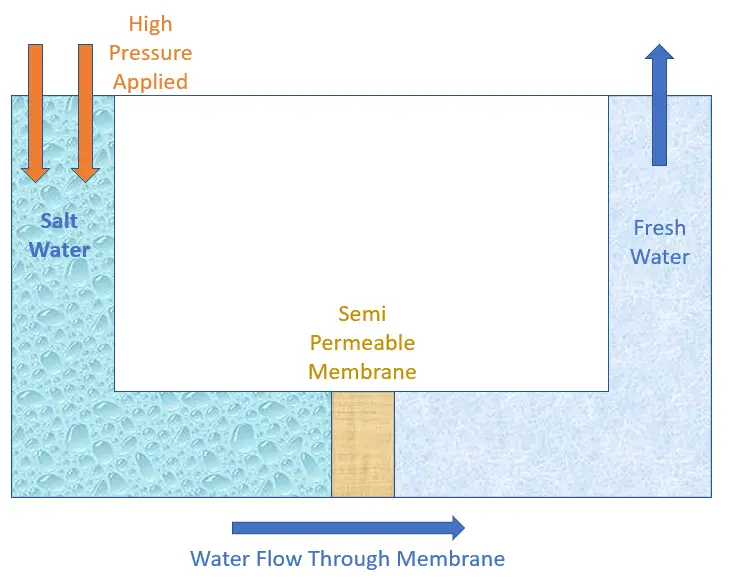
Reverse-Osmosis works by forcing salty water through a semi-permeable membrane removing impurities with fresh water flowing out the other side. The semi-permeable membranes in question have microscopic holes large enough to fit water molecules through but too fine for larger ions like Sodium, Magnesium, Chloride etc to pass through, thus making it similar to filtration but on a microscopic level. Large amounts of pressure are required to force the water through the membrane whilst leaving the salt behind as residue. This phenomenon that the high pressure is overcoming is known as “Osmotic Pressure” which is the natural flow of ions through a semi-permeable membrane from areas of high concentration to low concentration. Forcing sea water against this osmotic pressure can involve pressures of 100 to 800 bar to be efficient, however, Reverse-Osmosis will technically start to function at around 20 bar. Reverse-Osmosis usually works with the help of huge pumps churning out large volumes of water at crazy pressure for optimal efficiency however there are some potential tweaks in the process for varied applications.
Wave-powered desalination
Wave-powered desalination works by directly converting wave energy and the pressure it creates directly into the hydraulic pressure required to facilitate Reverse-osmosis, bypassing the need for large-scale electricity generation and pumps. These systems are generally more energy efficient as there is minimal energy loss from the source to the destination like with electricity generation.
Membrane distillation
Membrane distillation is a combination of osmosis and distillation. The process relies on a temperature difference across a membrane to evaporate water from a salty solution through a membrane where it condenses as water on the other side of the membrane and is then collected. Although not a widely used technique it is equally interesting in the way chemical properties are used to remove salt from the water.
Freeze-Thaw
Freeze-thaw desalination utilizes the effect of salt on water’s freezing temperature freezing to remove the salt from fresh water. Salt decreases the freezing temperature of the water so as salt water freezes, the frozen portion will contain progressively less salt, eventually leaving a salty brine in liquid form. This property is utilized by simply thawing frozen seawater that has allowed the brine to be removed leaving drinkable water. Although not an industrial process it highlights how both phase changes of water to and from solid and gas can be exploited to create clean water.

Electrodialysis membrane
Electrodialysis uses the difference in electric potential to remove salt from water. Water is moved through a pair of charged electronic membranes which attract and then separate salts in alternating channels with positive ions like Sodium flowing towards the anode and negative ions like Chloride flowing toward the cathode. This technique is particularly useful as it can be downsized quite effectively and was recently utilized by MIT to create a lightweight solar-powered desalination solution the size of a briefcase.
Microbial desalination
Microbial desalination uses cells of electro-active bacteria to remove salt from water in situ without having the transport the water anywhere. The cells function like biological electrochemical systems creating a natural anode and cathode gradient of the electro-active bacteria to draw anions and cations out of solution in a manner similar to a battery or capacitor. This is not really used as a standalone desalination treatment however it has been used as a pre-treatment for some processes including electrodialysis.

https://www.sciencedirect.com/topics/neuroscience/reverse-osmosis#:~:text=Reverse%20osmosis%20filtration%20uses%20high,microbiologic%20contamination%20and%20desalinate%20water.
https://www.appliedmembranes.com/reverse-osmosis-membranes-and-systems-for-low-tap-pressures.html
https://news.mit.edu/2022/portable-desalination-drinking-water-0428
https://www.sciencedirect.com/topics/engineering/multi-stage-flash-distillation
https://www.sciencedirect.com/topics/engineering/solar-distillation#:~:text=Solar%20distillation%20is%20a%20process,usually%20for%20small%2Dscale%20applications.
https://www.sciencedirect.com/science/article/pii/S2588912521000072
https://iasks.org/articles/ijtee-v15-i2-pp-103-110.pdf
https://www.sciencedirect.com/science/article/abs/pii/S1385894719316250


