A common query worldwide is how to remove oil from water. Removing petroleum-based products from water is not a simple process due to a variety of factors affecting the water and the various hydrocarbons that can end up as contaminants. The fact a one size fits all solution does not appear to exist on the topic inspired me to delve a bit deeper into the topic and come up with a general guide that is suitable for most queries on the topic. The main factors involved in deciding on the optimal solution are the level of contamination and the target purity of the water and oil post-separation.

Oil can be removed from water via a combination of physical separation due to differences in density, chemical additives like ozone which can help break down hydrocarbons or fractional distillation where differences in boiling points are exploited to separate the two products.
As there is no single approach to the problem of oil contamination in water, the guide is broken down into parts explaining the reason why oil is difficult to remove from water, five methods that can be utilized to extract oil from water, how the methods can be combined for an optimal solution and finally the challenges and complicating factors which can affect the process.
Why oil is difficult to remove from water.
Oil is difficult to remove from water because it is a liquid, so it can’t be filtered out nor can the water be boiled and collected as steam as oil also boils and will be collected alongside the water. Finally, oil doesn’t behave uniformly with reverse-osmosis membranes, leading to contamination and damage.
For the aforementioned reasons, oil is particularly difficult to remove from the water via standard means of filtration and purification, however, it does also possess some properties that lead to alternative removal techniques. Many of you may have forgotten the chemistry principles of molar polarity, particularly if you didn’t concentrate much in grade 10 chemistry, however, the important part to remember is that it is the reason why oils don’t dissolve in water. When you mix oil and water, they will always be separate blobs, kind of like vinegar and olive oil in salad dressing as they form what is known as an emulsion. The underlying reason behind molar polarity is quite technical so for the purposes of this article it isn’t necessary to go in-depth as long as you remember the key point that they won’t dissolve into one another. If you are interested there is a concise explanation of molar polarity available here.
Burning: Utilizing oil’s lower density and flammable nature.
Burning oil off the surface of water utilizes the fact that oil is typically flammable, it is insoluble in water and the fact that a lot of oils have lower specific gravity than water, meaning they float on the surface of the water.
Generally reserved for cases of extreme oil spillage like oil tankers running aground, burning oil off the surface is a quick response to immediate environmental threats, focussed more on minimizing damage rather than attaining a certain level of water purity. As most oils are less dense and subsequently lighter than water, when oil is spilt into a body of water it will generally hover on the surface, this combined with the fact that hydrocarbons (oil) are generally insoluble in water which means the oil floating on the surface is of sufficiently high purity to be able to burn if exposed to a naked flame.
I am sure you have all seen such scenes in Hollywood movies where oil is burning on the surface of the ocean after a plane or ship has crashed, whether purposeful or not, that phenomenon is utilizing the three properties of oil explained in this section. The level of water purity attained after burning is generally still extremely poor, however, oil removes a large percentage of the spilt oil in extreme instances which then allows for more precise methods mentioned in this article to further improve the water cleanliness.
Surface scraping: For targeting visible oil slicks.
Utilizing the density differential between oil and water along with oil’s insolubility in water, surface scraping is a method involving slowly dragging a rope or strip around a spilt body of oil to either contain, concentrate or move the spilt hydrocarbons.
Often used in conjunction with the burning of oil slicks, surface scraping is a simple and low-impact technique of damage control after there has been a spill or accident involving hydrocarbons and water. The technique behind the process is quite simple, a floating rope or strip is laid out around an oil slick before the strip is either towed (if moving) or simply shortened if being concentrated and/or collected.
Decanting: Utilizing differences in density to remove oil from water.
Decanting oil from the water’s surface is generally used in small bodies of water like tanks or ponds, where the oil is given time to float to the surface of the water before being carefully poured or pumped from the surface into a neighbouring tank or vessel.
Although quite simplistic in nature, decanting oil from the surface of the water can attain quite an impressive level of purity. Multiple settling tanks or vessels are used to create a series of decanting and settling tanks, each with a steadily increasing level of purity for both oil and water. For instance, consider the following heavily simplified scenario:
Initial water is 10% oil, the liquid decanted to the next tank is 75% oil and then a similar improvement in purity again at the next tank and so on, leading to oil purities of over 99% if performed enough times!
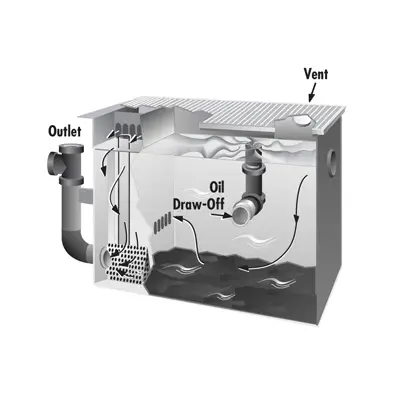
This technique has been utilized in locations like restaurants where considerable amounts of grease and oil are flushed down the drain, in order to avoid environmental damage and blocked pipes, restauranteurs are incentivized to capture as much oil as possible to then be disposed of in specialized locations. Decanting systems are installed to collect such contaminated wastewater, and extract the majority of oil from the water before releasing the water to the sewerage or septic system.
There are some efficient commercially available systems that effectively separate oil and grease from water, one such company operating out of Illinois called Rockford Separators that have a great following in the area, particularly in the restaurant industry due to their compact nature and relatively high efficiency. An example of how their systems work is displayed on the right, they can also engineer systems for every situation, and inventions like this truly do help out with improving the quality of water runoff.
Ozonation: Oxidizing hydrocarbons into oxygen, hydrogen and carbon
Ozone breaks down Hydrocarbons (Oil & Grease) into water (H2O), oxygen (O2) and carbon dioxide (Co2).
The hydrocarbons which are generally made of a combination of Carbon, Hydrogen and Oxygen atoms are broken down by ozone oxidizing the compounds leading to the breakdown of the strong covalent bonds between the atoms. Once the hydrocarbons are broken down into carbon dioxide, water and oxygen they are easily removed from the solution via natural means. Ozone is efficient at breaking down all BTEX hydrocarbons (Benzene, Toluene, Ethylbenzene, Xylene) these are common hydrocarbons found in crude oil.
An example of how Benze is broken down when oxidized by ozone is highlighted below:
C6H6 + 11 O3 —- > 6Co2 + 3 H2O + 11 O2
Perhaps the important fact about ozone and what sets it apart from other effective treatment methods like reverse osmosis is its ability to break down hydrocarbons without any risk to its functionality. The way ozone breaks down dissolved hydrocarbons efficiently utilizes its powerful oxidation properties in a similar manner to the way it brings metals out of solution.
Although good at removing most common hydrocarbons, ozone is not 100% effective with all oil-based compounds, so ozone can be considered an important stage in the process of creating drinking water from contaminated sources or a standalone treatment method for lower quality uses like industrial process water or even some forms of agriculture.
Fractional Distillation: Utilizing different boiling points to separate solvents.
Fractional distillation is more commonly associated with alcohol production, where a solution of alcohol and water is held beneath the water’s boiling point (100 °C) but above that of alcohol (78.37 °C) allowing the alcohol to be boiled off and collected via condensation in the form of pure alcohol.
The same theory applies to hydrocarbons although, with an additional step to extract pure water, most hydrocarbons boil at temperatures lower than 100 °C, so they are initially boiled off and collected, however, the remaining solution still can contain some particular hydrocarbons with water.
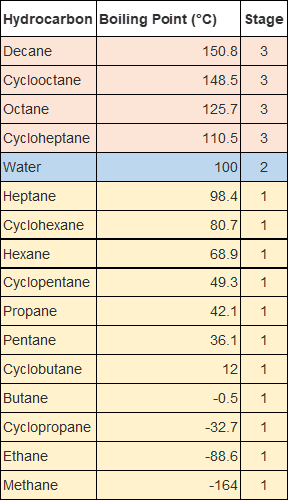
In order to ensure water is separated from a contaminated mix of hydrocarbons, assuming the worst-case scenario as displayed in the table on the left, three stages of fractional distillation would be required.
The first stage would involve heating the solution to around 99°C and holding the solution there for a considerable amount of time until it can be safely assumed that the stage 1 hydrocarbons have been removed. The next stage would involve heating the liquid to somewhere between 100 °C and 110 °C to enable the collection of water, once again, this would be held for sufficient time, before turning the heat off. After this process, you would be left with three liquids, one containing stage one hydrocarbons, one containing water and the stage 3 hydrocarbons remaining in the initial vessel.
As you can probably imagine, the aforementioned process is not something that is extremely practical and would only really be utilized in times of very particular water quality requirements, like human or animal consumption. It is good to know though, that boiling point differences can be effectively utilized to separate hydrocarbons from water if absolutely required!
Centrifugal Force: Utilize density differentials.
Using centrifugal force to separate fluids of differing densities is performed by spinning the combined fluids together, forcing the denser of the fluids to the outer edge where it can be captured. In the case of water and oil, water is forced to the outer edge where it will be split off from the oil. Although this could technically work to remove oil from water, it is generally used where water is seen as the contaminant in oil just due to the small size of most centrifuges meaning the contaminated products to be processed will usually be of lower volume like water in engine oil for instance. Centrifugal separation is also very effective at removing solids from oil, such as metal fragments which are extremely dense and rapidly forced to the outer edge of the centrifuge.

Combinations to convert heavily polluted to drinking water.
As could be assumed, most scenarios involving the removal of oil from water require a combination of the aforementioned processes. Starting at the top of the list with burning surficial oil after some large-scale disaster right down to using fractional distillation or centrifugal separation to really fine-tune the final target product, be it oil or water.
Generally speaking, most scenarios the average reader of this article will encounter could probably be solved with the use of a decanting system followed by either fractional distillation or centrifugal separation, depending on your target product. The decanting systems are very effective at removing a large portion of the contamination before being fine-tuned with a more intricate separation technique.
The use of ozone is very practical for high-flow applications due to its scalable nature, this leads to it being utilized globally for a variety of industrial and municipal level water treatment programs, it is the only large-scale technique that is genuinely recognized to break down hydrocarbons and heavy minerals in addition to bacteria and microorganisms. If you are interested in reading further about ozone, I encourage you to take a look at my article on the topic What is ozonation, and how does it help purify water?
Sources
https://www2.chem.wisc.edu/deptfiles/genchem/netorial/rottosen/tutorial/modules/intermolecular_forces/01review/review5.htm
https://www.rkfdseparators.com/oil-separators/
https://www.sciencedirect.com/topics/chemistry/alkane
https://letsdowater.com/what-is-ozonation-and-how-does-it-help-purify-water/


